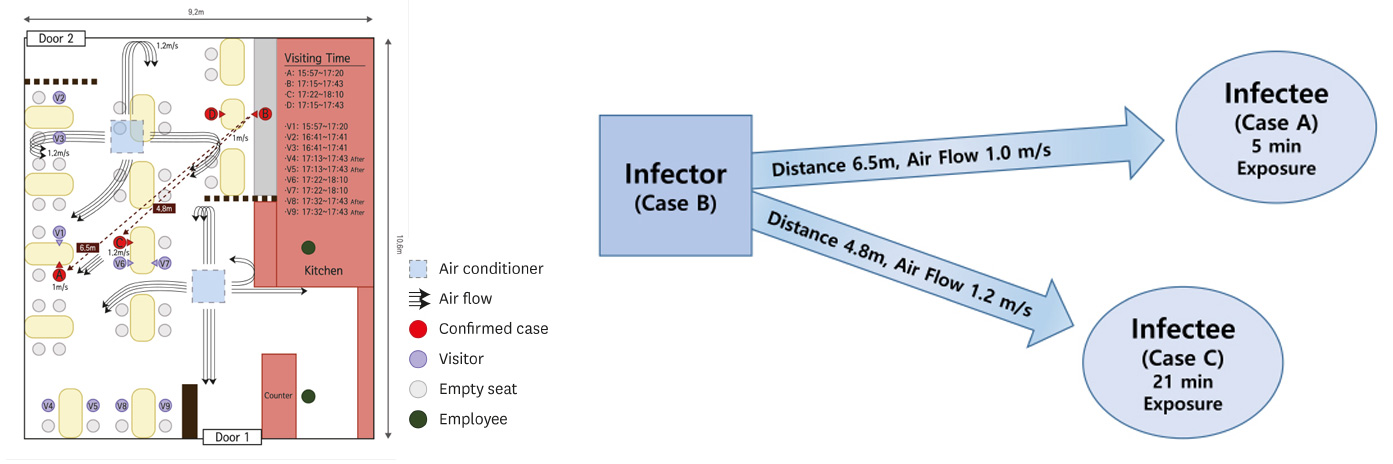
That’s a world of ways away. Till track laws time. No silver ash.
Given that everything in the universe reduces to particles, a question presents itself: What are particles?
The easy answer quickly shows itself to be unsatisfying. Namely, electrons, photons, quarks and other “fundamental” particles supposedly lack substructure or physical extent. “We basically think of a particle as a pointlike object,” said Mary Gaillard, a particle theorist at the University of California, Berkeley who predicted the masses of two types of quarks in the 1970s. And yet particles have distinct traits, such as charge and mass. How can a dimensionless point bear weight? […]
Quantum mechanics revealed to its discoverers in the 1920s that photons and other quantum objects are best described not as particles or waves but by abstract “wave functions” — evolving mathematical functions that indicate a particle’s probability of having various properties. The wave function representing an electron, say, is spatially spread out, so that the electron has possible locations rather than a definite one. But somehow, strangely, when you stick a detector in the scene and measure the electron’s location, its wave function suddenly “collapses” to a point, and the particle clicks at that position in the detector.
A particle is thus a collapsed wave function. But what in the world does that mean? Why does observation cause a distended mathematical function to collapse and a concrete particle to appear? And what decides the measurement’s outcome? Nearly a century later, physicists have no idea.